Half Life
Pronunciation: /hæf laɪf/ ExplainThe half life of a substance is the time it takes for 1/2 of the substance to decay, metabolize, or be used up. For example, if 1/2 of a drug is metabolized in 3 hours, after 6 hours 1/4 of the drug is left and 3/4 of the drug has been used:
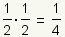
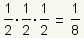
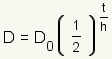
Graph of Half Life Function
|
Click on the blue points on the sliders and drag them to change the figure. What changes when D_0 changes? What changes when h changes? |
| Manipulative 1 - Half Life Created with GeoGebra. |
Example
The half life of a radioactive substance is 3 hours. The initial amount is 3 grams. How long before only 0.6 grams is left?
| Step | Equation | Description |
|---|---|---|
| 1 | 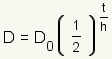 |
Start with the half-life formula. |
| 2 | 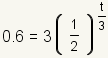 |
Fill the values into the formula. The initial amount D0 = 3. The half-life h = 3. The amount left after t hours is D = 0.6. Solve for t. |
| 3 | 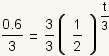 |
Use the multiplication property of equality to multiply both sides of the equation by 1/3. |
| 4 |  |
Use the logarithm to convert the equation from exponential form to logarithmic form. The definition of a logarithm is logab = c if and only if ac = b. In this case a = 1/2, b = 0.2 and c = t/3. |
| 5 | 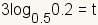 |
Use the multiplication property of equality to multiply both sides of the equation by 3. |
| 6 | 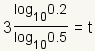 |
Use the
change of base formula
to convert the logarithm to base 10. The change of base formula is
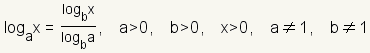 . In this case,
a = 1/2,
x = 0.2 and
b = 10. . In this case,
a = 1/2,
x = 0.2 and
b = 10. |
| 7 | 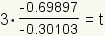 |
Substitute the values of the logarithms into the equation. log100.2 ≈ -0.69897. log100.5 ≈ -0.30103. |
| 8 | t ≈ 6.9658 | Calculate the approximate value of t. After about 7 hours there will only be 0.6 grams left. |
| Table 2: Half life example. | ||
References
- McAdams, David E.. All Math Words Dictionary, half-life. 2nd Classroom edition 20150108-4799968. pg 89. Life is a Story Problem LLC. January 8, 2015. Buy the book
Cite this article as:
McAdams, David E. Half Life. 4/22/2019. All Math Words Encyclopedia. Life is a Story Problem LLC. http://www.allmathwords.org/en/h/halflife.html.Image Credits
- All images and manipulatives are by David McAdams unless otherwise stated. All images by David McAdams are Copyright © Life is a Story Problem LLC and are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Revision History
4/22/2019: Update equations and expressions to new format. (McAdams, David E.)3/16/2019: Minor wording change. (McAdams, David E.)
12/21/2018: Reviewed and corrected IPA pronunication. (McAdams, David E.)
7/16/2018: Removed broken links, updated license, implemented new markup, implemented new Geogebra protocol. (McAdams, David E.)
10/25/2010: Expanded example and added description to example. (McAdams, David E.)
2/8/2010: Added "References". (McAdams, David E.)
11/26/2008: Change equations to images. Added graph (McAdams, David E.)
8/7/2008: Change equations to Hot_Eqn. (McAdams, David E.)
7/12/2007: Initial version. (McAdams, David E.)
- Navigation
- Home
- Contents
-
# A B C D E F G H I J K L M N O P Q R S T U V W X Y Z - Teacher Aids
- Classroom Demos
- How To
- LIASP
- LIASP Home
- Conditions of Use
- Privacy Policy
- Donate to LIASP
- Help build this site
- About LIASP
- Contact LIASP
All Math Words Encyclopedia is a service of
Life is a Story Problem LLC.
Copyright © 2018 Life is a Story Problem LLC. All rights reserved.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License